-
 Home
Home
-
 News
News
Latest Educational News Stories
Daily update of all national, international news, picture stories, college / university announcements and educational events.
-
 Colleges
Colleges
Pakistan's Largest Database of Colleges and Universities
Explore Largest Directory of Private and Govt. Colleges, Universities and find best institute for your future Education.
-
 Courses
Courses
-
 Admission
Admission
-
 Lectures
Lectures
-
 Online Test
Online Test
Short Question
- 9th Class Physics Short Questions
- 9th Class Chemistry Short Questions
- 9th Class Math Short Questions
- 9th Class Biology Short Questions
- 9th Class Computer Short Questions
- 9th Class English Short Questions
- 10th Class Physics Short Question
- 10th Class Chemistry Short Question
- 10th Class Math Short Question
- 10th Class Biology Short Question
- 10th Class Computer Short Question
- 10th Class English Short Question
-
 Past Papers
Past Papers
-
 Date Sheets
Date Sheets
-
 Results
Results
Exam Results 2024
Check online Results 2024 Matric Inter BA BSc B.Com MA MSc M.Com CSS PCS MCAT ECAT of all educational boards and universities in Pakistan
-
 Study Abroad
Study Abroad
Study Abroad Programs and Opportunities for Pakistani Students
Explore free study abroad search to find programs, consultants, events to study in USA, UK, Australia, China, Malaysia and many others.
-
 Jobs
Jobs
-
 Tutors
Tutors
-
 More
More
-
 Apps
Apps
MCQ's Test For ECAT Chemistry Chapter 8 Chemical Equilibrium
Try The MCQ's Test For ECAT Chemistry Chapter 8 Chemical Equilibrium
-
Total Questions30
-
Time Allowed30
Question # 1
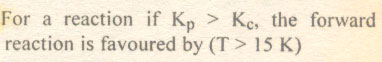
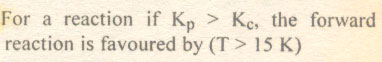
Question # 2
A chemical reaction A---------->B is said to be in equilibrium when :
Question # 3
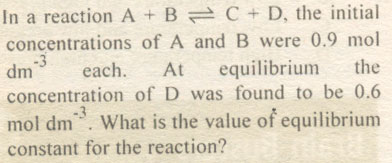
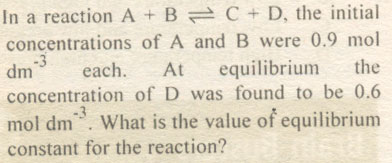
Question # 4


Question # 5
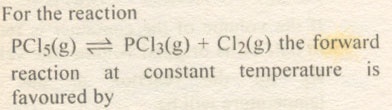
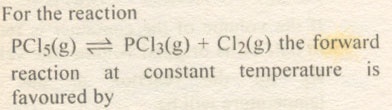
Question # 6
Which one of the following has no units of its Kcvalue
Question # 7
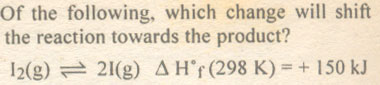
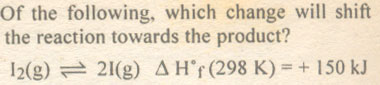
Question # 8
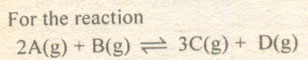
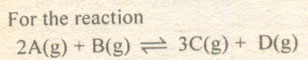
Question # 9
2SO2+ O2⇌2SO2H= 188KJ mole-1
Which statement about following equilibrium is correct :
Question # 10
A solution having pH = 4 its OH-ion concentration in mole dm-3is
Question # 11
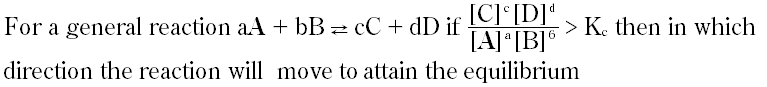
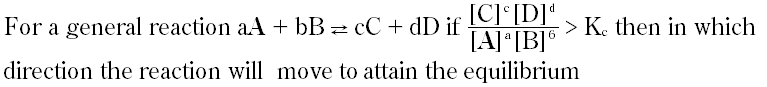
Question # 12
pKb value of NH4OH is 4.74. If the concentration of NH4OH is 1 molar containing 0.1 molar NH4Cl, then pH of this buffer will be
Question # 13
H2 + L2 ----2Hl
H2 + L2 ----2HlIn the above equilibrium system, if the concentration of reactants at 25°C is increased, the value KC will :
Question # 14
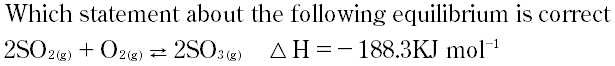
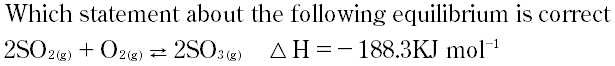
Question # 15
The ionization constant of an acid is expressed in term of the following constant
Question # 16


Question # 17
A buffer solution of 0.1 molar HCOOH and 0.1 molar HCCONa has pH = 3.78 To is 0.01 molar HCl is added, then pH of the buffer solution becomes
Question # 18
The state of equilibrium refers to
Question # 19
The correct relation b/wKc andKp is :
Question # 20
1 mole of N2and 2 moles of H2are allowed to react in a 1 dm3vessel. At equilibrium 0.8 mole of NH3is formed. The concentration of H2in the vessel is
Question # 21
The rate of forward reaction is two times that of the reverse reaction at a given temperature and identical concentration, K equilibrium is
Question # 22
The solubility product of
AgCl is 2.0 x 10-3 mol2 dm-6 , The maximum
concentration of Ag ion in the solution is :
Question # 23
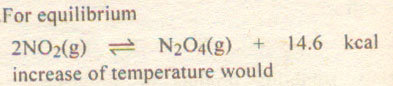
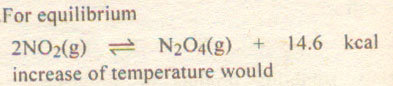
Question # 24
When a weak acid is dissolved in water or a weak base dissolved in water, then in both cases the conjugate acid base pair is produced. The ionization constants Kaand Kbof a pair are related with each other as
Question # 25


Question # 26
Kavalue of HF acid is 6.7 x 10-15the acid is a
Question # 27
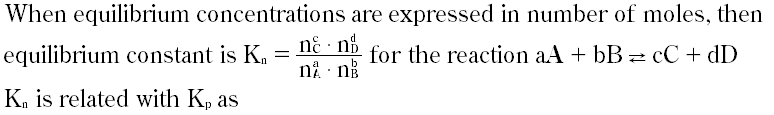
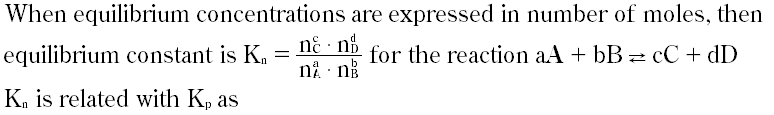
Question # 28
Buffers having pH less than 7 are made
Question # 29
ph of the buffer CH3COOh + CH3COONa is 3.76. If the mixture contains 1 molar acetic acid and 0.1 molar sodium acetate, then pKa of this buffer is
Question # 30
The rate at which a substance reacts is directly proportional to its active mass and the rate of reaction is directly proportional to the product of the active masses of reacting substances, is called
Top Scorers Of ECAT Chemistry Chapter 8 Chemical Equilibrium MCQ`s Test
-
A Arian Ahmed 06 - Mar - 2024 00 Min 04 Sec 115/120 -
H Huzaifa Asim 28 - May - 2024 01 Min 12 Sec 115/120 -
M Muneeb Aslam 03 - Jun - 2024 09 Min 57 Sec 105/120 -
? ?GøÐ øf ?ë??h? 29 - May - 2024 32 Min 31 Sec 95/120 -
H Hamza Malhi 10 - Mar - 2024 14 Min 42 Sec 85/120 -
M Maryam Fatima 19 - Mar - 2024 09 Min 42 Sec 80/120 -
F Fatima Tabassum 16 - Jan - 2025 09 Min 48 Sec 65/120 -
E Essa raza 22 - Jun - 2024 09 Min 07 Sec 55/120 -
K kayani 21 - Jul - 2024 09 Min 41 Sec 55/120 -
T Tamshi Sohail 20 - Apr - 2024 23 Min 19 Sec 55/120 -
R random 11 - May - 2024 06 Min 15 Sec 50/120 -
H Haniya Ali 14 - Mar - 2024 14 Min 41 Sec 50/120 -
E Ezzah Ansari 15 - May - 2024 15 Min 25 Sec 50/120 -
M Meena 19 - Apr - 2024 10 Min 19 Sec 45/120 -
U Usman Irshad 04 - Mar - 2024 12 Min 29 Sec 45/120
ECAT Chemistry Chapter 8 Important MCQ's
| Sr.# | Question | Answer |
|---|---|---|
| 1 | pKb value of NH4OH is 4.74. If the concentration of NH4OH is 1 molar containing 0.1 molar NH4Cl, then pH of this buffer will be |
A. 3.74
B. 10.26
C. 4.74
D. 9.26
|
| 2 |
2SO2 + O2 ⇌ 2SO2 H= 188KJ mole-1 |
A. The value ofKpfalls with arise in temperature.
B. The value ofKpis equal tokc.
C. The value ofKpfalls with the increase pressure.
D. Adding V2O5 catalyst increase the equilibrium
yield of Sulphur trioxide.
|
| 3 |

|
A. Increase in concentration of 1
B. Decrease in concentration of I2
C. Increase in temperature
D. Increase in total pressure
|
| 4 | For which system does the equilibrium constant, KC has units of concentration | |
| 5 |
N2 + 3H2 ⇌2NH3 The unit of Kc for tis reaction will be: |
A.
mol2 dm-6
B.
mol-2 dm+6
C.
mol dm-3
D.
mol-1 dm+3 |
| 6 | If pH of buffer of 1 mole dm-3of HCOOH + 0.1 mole dm-3HCOONa having pKa = 3.78 is |
A. 1.78
B. 2.78
C. 3.78
D. 4.78
|
| 7 |

|
A. 32
B. 64
C. 16
D. 4
|
| 8 | Kbfor NH4OH is 1.81 x 10-5, then Kavalue of its conjugate base is |
A. 1.81 x 10+5
B. 1.81 x 10-9
C. 5.5 x 10-9
D. 5.5 x 10-10
|
| 9 |
Which statement about the following equilibrium in correct? 2SO2 (g)+ O2(g)---------------2sO3(g)H= - 188.3 KJ mol-1 |
A. T value of Kp falls witha rise in temperate.
B. The value of Kp falls withincreasing pressure
C. Adding V2O5catalyst increase the equilibrium yield of sulfur trioxide
D. The value of Kp is equal toKp
|
| 10 |

|
A. Shift reaction toward forward direction
B. Shift reaction backward
C. Lower the value of Kc
D. No change in reaction
|








.jpg)







