-
 Home
Home
-
 News
News
Latest Educational News Stories
Daily update of all national, international news, picture stories, college / university announcements and educational events.
-
 Colleges
Colleges
Pakistan's Largest Database of Colleges and Universities
Explore Largest Directory of Private and Govt. Colleges, Universities and find best institute for your future Education.
-
 Courses
Courses
-
 Admission
Admission
-
 Lectures
Lectures
-
 Online Test
Online Test
Short Question
- 9th Class Physics Short Questions
- 9th Class Chemistry Short Questions
- 9th Class Math Short Questions
- 9th Class Biology Short Questions
- 9th Class Computer Short Questions
- 9th Class English Short Questions
- 10th Class Physics Short Question
- 10th Class Chemistry Short Question
- 10th Class Math Short Question
- 10th Class Biology Short Question
- 10th Class Computer Short Question
- 10th Class English Short Question
-
 Past Papers
Past Papers
-
 Date Sheets
Date Sheets
-
 Results
Results
Exam Results 2024
Check online Results 2024 Matric Inter BA BSc B.Com MA MSc M.Com CSS PCS MCAT ECAT of all educational boards and universities in Pakistan
-
 Study Abroad
Study Abroad
Study Abroad Programs and Opportunities for Pakistani Students
Explore free study abroad search to find programs, consultants, events to study in USA, UK, Australia, China, Malaysia and many others.
-
 Jobs
Jobs
-
 Tutors
Tutors
-
 More
More
-
 Apps
Apps
MCQ's Test For ECAT Chemistry Chapter 1 Basic Concepts
Try The MCQ's Test For ECAT Chemistry Chapter 1 Basic Concepts
-
Total Questions30
-
Time Allowed30
Question # 1
Which one of the following statements is not correct
Question # 2
The percentage of which element in the organic compound is determined by the difference method
Question # 3
The number of subatomic particles in atoms sidcovered is more than:
Question # 4
The relative abundance of Pb isotopes is 1.5% Pb204, 23.6% Pb206, 22.6% Pb207, 52.3% Pb208The relative atomic mass of Pb is
Question # 5
C6H12O6and C12H22O11 are:
Question # 6
The mass of one mole of proton is
Question # 7
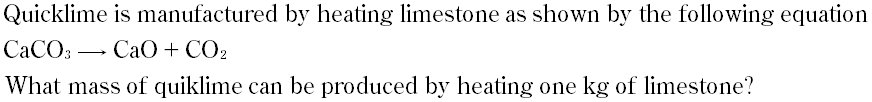
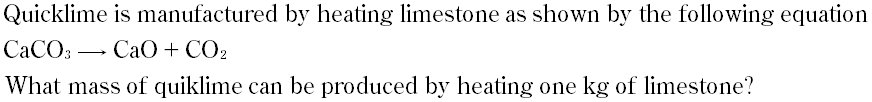
Question # 8
Macromolecules are
Question # 9
Which of the following statements is not true?
Question # 10
A limiting reactant is one which according to the stoichiometric equation
Question # 11


Question # 12
The number of isotopes of gold is
Question # 13
CL2, N2 and O2 are:
Question # 14
Which one of the following compounds does not have the empirical formula CH2O?
Question # 15
Which of the following compounds contains the highest percentage by mass of nitrogen?
Question # 16
One of the following statements is incorrect
Question # 17


Question # 18
First atomic theory was put forward by an English school teacher:
Question # 19
The diameter of atoms is of the order:
Question # 20
CO+ is an example of
Question # 21
Who one mole of each of the following is completely burned in oxygen, which gives the largest mass of carbon dioxide?
Question # 22


Question # 23
Matter is defined as any thing which occupies space and:
Question # 24
The wave length of visible light is 500 nm. In S.I. unit this value is
Question # 25
Which statement about molecule is incorrect ?
Question # 26
Isotopes differ in
Question # 27
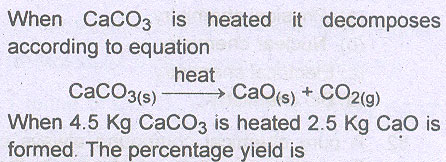

Question # 28
Covalent compound s mostly exist in the form of:
Question # 29
Determination of atomic masses and invention of system of writing symbols was made by:
Question # 30
Hemoglobin is 68000 times heavier than:
Hemoglobin is 68000 times heavier than:
Top Scorers Of ECAT Chemistry Chapter 1 Basic Concepts MCQ`s Test
-
F Fatima Tabassum 09 - Jan - 2025 04 Min 47 Sec 100/120 -
M M Umer Arain 01 - Nov - 2024 00 Min 10 Sec 95/120 -
Z zakee 09u 28 - Nov - 2024 18 Min 56 Sec 95/120 -
A Aroosha Tariq 13 - Nov - 2024 06 Min 02 Sec 90/120 -
Z Zainab Tanvir 07 - Dec - 2025 09 Min 10 Sec 80/120 -
I ISI?Shadow 16 - Nov - 2024 17 Min 47 Sec 80/120 -
N notghouri playz 13 - Dec - 2024 27 Min 04 Sec 80/120 -
T Tayyaba Attique 16 - Nov - 2024 07 Min 22 Sec 75/120 -
G Ghazi Imam 03 - Nov - 2024 18 Min 03 Sec 75/120 -
U umerasif 14 - Nov - 2024 105 Min 42 Sec 75/120 -
A agha hamza 22 - Nov - 2025 22 Min 17 Sec 72/120 -
A abdullah masood 04 - Dec - 2024 10 Min 54 Sec 70/120 -
M Muhammad Awais 01 - Jan - 2025 05 Min 43 Sec 65/120 -
A Aqsa afridi 02 - Dec - 2024 24 Min 01 Sec 65/120 -
A afraz afraz 17 - Jan - 2025 00 Min 39 Sec 60/120
ECAT Chemistry Chapter 1 Important MCQ's
| Sr.# | Question | Answer |
|---|---|---|
| 1 | Al3+is a symbol for aluminium |
A. Atom
B. Ion
C. Cation
D. Anion
|
| 2 | One of the following statements is incorrect |
A. Actual yeild is always less than the theoretical yield
B. The formula of a compound is not definite
C. Law of conservation of mass is applied in stoictiometry
D. Boyles law is applied in stoichiometry
|
| 3 |

|
|
| 4 | Relative atomic mass of an element is the mass of the element relative to |
A. 1/12 mass of carbon-12
B. 1/12 mass of carbon
C. 1 mass of hydrogen atom
D. 1/16 mass of oxygen
|
| 5 | Which of the following compounds contains the highest percentage by mass of nitrogen? |
A. Ammonia, NH3
B. Ammonium carbamate, NH2CO2NH4
C. Ammonium carbonate, (NH4)2CO3
D. Hydrazine, N2H4
|
| 6 |

|
A. 8 g
B. 16 g
C. 32 g
D. 24 g
|
| 7 | The quantitative relationship between the substances according to balanced equation describes |
A. Reversible reactions
B. Stoichiometry
C. Limiting reacting
D. Percentage composition
|
| 8 | The mass of one mole of proton is |
A. 1.008 g
B. 0.184 g
C. 1.673 g
D. 1.008 mg
|
| 9 | CO+ is an example of |
A. Stable molecule
B. Anionic molecule ion
C. Cationic molecular ion
D. Free radical
|
| 10 | Which one of the following statements is not correct |
A. A molecule is the smallest particle of an element which can exist independently
B. He is a molecule of helium
C. S8is a molecule of sulphur
D. O3is a molecule of oxygen
|








.jpg)







